An intersocietal panel of experts in CT convened by the American Association of Physicists in Medicine (AAPM)—with representation from clinical practice, academia, and industry input from Siemens Healthineers and Canon—examined a new performance measure in the quality-based payment programs of the Centers for Medicare & Medicaid Services (CMS). Publishing their findings in the American Journal of Roentgenology [1], the panel identified 20 important issues and ambiguities with the new measure, which became effective this year.
Collectively, these issues reflect unclear definitions, opaque methodologies, technical and legal barriers, and potential misalignment with clinical realities—posing significant obstacles to consistent, equitable, and scientifically valid implementation across diverse care settings.
Ambiguity surrounds where reporting is required versus optional and exactly which adult study types qualify, compounded by difficulties in consistent inpatient versus outpatient categorization. Terminology inconsistencies and unclear mapping of studies to dose and image quality categories add to the confusion. Meanwhile, patient size assessment methodology and calculation of size-adjusted dose diverge from established standards, while noise measurement lacks a recognized protocol. Criteria for excluding studies and handling combination studies remain undefined.
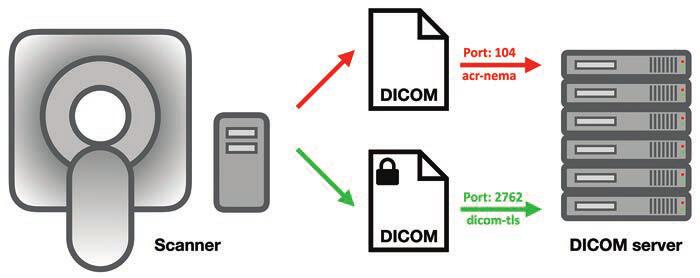
Then, there are the tech queries: is HL7 EHR connectivity mandatory, are alternative data transfer mechanisms even feasible, what potential IT burdens and/or security liabilities will radiology practices have to shoulder? Also, performance expectations for compliance thresholds are unspecified, as are methods for comparing diverse protocols under a single set of thresholds. Identical thresholds across different categories raise additional questions.
“Transparency and stakeholder engagement are essential for effective quality initiatives in medicine,” said Mahadevappa Mahesh, MS, PhD, president of AAPM.

Dr. Mahesh | President, AAPM
“We wrote this paper to call attention to issues and ambiguities with the CMS measure, and we look forward to working with CMS to address these issues and continue the culture of quality and safety that has developed in CT imaging over the past two decades.”
Balancing Image Quality and Patient Safety
One of the benefits to patients that will come from “The New CMS Measure of Excessive Radiation Dose or Inadequate Image Quality in CT: Issues and Ambiguities—Perspectives from an AAPM-Commissioned Panel” in AJR is that the expertise of the entire imaging community will be used to develop quality improvement initiatives that will keep radiation doses as low as possible while maintaining the quality of medically essential CT imaging. From physicians and physicists to technologists, regulators, and business leaders, “we’re confident that we can get this right by working together,” said Dr. Mahesh.
Technology Has Already Lowered Doses
A lifesaving technology used to diagnose disease and guide treatment, CT is the first-line imaging technique in many cases, especially in emergency departments and cancer centers. Concerns have been raised about the increased utilization of CT in medicine because the modality uses ionizing radiation, which at very high doses is known to increase a patient’s risk for developing cancer. However, at the low doses of radiation utilized in medical imaging, including in CT, the risk is extremely small—perhaps negligible.
Over the past two decades, imaging and allied health professionals have collectively worked to reduce CT doses. New scanner technologies have played a starring role in decreasing doses, including features that automatically measure the size of the patient and adjust the radiation dose to the right value. This is especially important for children, who require lower doses than adults due to their smaller size.

Dr. McCollough | Prior President, AAPM
“Some authors multiply the very small potential risk of a CT scan by the millions of patients who receive one and predict that we will see an increase in cancer,” said Cynthia McCollough, PhD, past president of AAPM.
“This can lead to alarmist stories and patients who really need a CT refusing to get one. Further, at the low doses we are talking about, it is debated whether the risk is even real. CT has been around for over 50 years and the predicted increases in cancer just aren’t being seen.”
Editorials Stress Ticking Clock, Call for Clarity
In her accompanying AJR editorial, Stephanie Leon, PhD, of the University of Florida in Gainesville, noted that “quality-based payment programs will be impacted starting in January 2027,” which means that imaging has two years and counting to figure all of this out [2].
| CMS Quality Reporting Program | CMS Payment System | Reporting Requirement | Timeline |
| Hospital IQR Program | HIPPS | Optional. Hospitals are required to report three eCQMs self-selected from a list and three eCQMs mandated by CMS. The measure will be available on the self-selection list and thus its reporting is optional. | Reporting will begin in CY 2025; CY 2025 results will impact FY 2027 payments. |
| Hospital OQR Program | HOPPS | Required. Once the measure is fully implemented, hospitals will be required to report the measure. | Reporting will be voluntary in CY 2025 and mandatory in CY 2027; CY 2027 performance will impact CY 2029 payments. |
| MIPSᵃ | MPFS | Optional. Participants are required to report six MIPS quality measures, including at least one outcome measure, that are self-selected from a list (possibly a specialty-defined measure set depending on the reporting mechanism). If more than six measures are available, then reporting the measure is optional. | Reporting will begin in CY 2025; CY 2025 results will impact FY 2027 payments. |
IQR: Inpatient Quality Reporting; OQR: Outpatient Quality Reporting; MIPS: Merit-based Incentive Payment System; HIPPS: Hospital Inpatient Prospective Payment System; HOPPS: Hospital Outpatient Prospective Payment System; MPFS: Medicare Physician Fee Schedule; eCQM: electronic clinical quality measure; CY: calendar year; FY: fiscal year
aApplies to clinicians and clinician groups
Another AJR editorial written by Kishore Rajendran, PhD, of the Mayo Clinic in Rochester, MN, and chair of the working group on the physics of quantitative imaging at AAPM, called for improved transparency, too. “A nonproprietary, community-based approach is imperative to ensure full transparency, achieve consensus among CT stakeholders, and provide reliable clinical diagnoses at the lowest radiation dose possible,” wrote Dr. Rajendran [3].
Watch as AJR senior author Ehsan Samei, PhD, and first author Jered R. Wells, PhD, call for a fundamental shift toward open-source, open-access, consensus-based, and community-owned strategies and resources to ensure quality and safety of CT: YouTube.com/@AJR_Radiology
References:
- Wells JR, Christianson O, Gress D, et al. The new CMS measure of excessive radiation dose or inadequate image quality in CT: issues and ambiguities—perspectives from an AAPM-commissioned panel. AJR 2025 May. doi: 10.2214/AJR.24.32458
- Leon, SM. CMS measure on CT dose and image quality: good intentions, but not quite ready for prime time. AJR 2025 May. doi: 10.2214/AJR.25.32908.
- Rajendran K. Transparency and stakeholder engagement as cornerstones for effective quality initiatives in medical imaging. AJR May. doi: 10.2214/AJR.25.32859